United States Core Data for Interoperability (USCDI)
The United States Core Data for Interoperability (USCDI) is a standardized set of health data classes and constituent data elements for nationwide, interoperable health information exchange. Review the USCDI Fact Sheet to learn more.
A USCDI Data Class is an aggregation of Data Elements by a common theme or use case.
A USCDI Data Element is a piece of data defined in USCDI for access, exchange or use of electronic health information.
USCDI ONC New Data Element & Class (ONDEC) Submission System
USCDI V1
Please reference the USCDI version 1 document to the left for applicable standards versions associated with USCDI v1.
Harmful or undesired physiological responses associated with exposure to a substance.
Health professional’s conclusions and working assumptions that will guide treatment of the patient.
Information about a person who participates or is expected to participate in the care of a patient.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Desired state to be achieved by a patient.
Health related matter that is of interest, importance, or worry to someone who may be the patient, patient’s family or patient’s health care provider.
Record of vaccine administration.
Analysis of clinical specimens to obtain information about the health of a patient.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Data used to categorize individuals for identification, records matching, and other purposes.
Activity performed for or on a patient as part of the provision of care.
The metadata, or extra information about data, regarding who created the data and when it was created.
Representing a patient’s smoking behavior.
Unique identifier(s) for a patient's implantable device(s).
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
USCDI V2
The USCDI v2 contains data classes and elements from USCDI v1 and new data classes and elements submitted through the ONDEC system. Please reference the USCDI Version 2 document to the left for applicable vocabulary standards versions associated with USCDI v2 and to the ONC Standards Bulletin 21-3 for more information about the process to develop USCDI v2 and future versions.
Harmful or undesired physiological responses associated with exposure to a substance.
Health professional’s conclusions and working assumptions that will guide treatment of the patient.
Information about a person who participates or is expected to participate in the care of a patient.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Non-imaging and non-laboratory tests performed that result in structured or unstructured findings specific to the patient to facilitate the diagnosis and management of conditions.
Tests that result in visual images requiring interpretation by a credentialed professional.
Information related to interactions between healthcare providers and a patient.
Desired state to be achieved by a patient.
Health related matter that is of interest, importance, or worry to someone who may be the patient, patient’s family or patient’s health care provider.
Record of vaccine administration.
Analysis of clinical specimens to obtain information about the health of a patient.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Data used to categorize individuals for identification, records matching, and other purposes.
Condition, diagnosis, or reason for seeking medical attention.
Activity performed for or on a patient as part of the provision of care.
The metadata, or extra information about data, regarding who created the data and when it was created.
Representing a patient’s smoking behavior.
Unique identifier(s) for a patient's implantable device(s).
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
USCDI V3
Please read the USCDI v3 standard document and the ONC Standards Bulletin 22-2 for details. Consistent with EO 14168 and OPM guidance, ASTP/ONC is exercising enforcement and issuing certification guidance for the ONC Health IT Certification Program with respect to certain data elements in USCDI v3. For more information see https://www.healthit.gov/topic/uscdi-v3-data-elements-enforcement-discretion.
Harmful or undesired physiological responses associated with exposure to a substance.
Health professional’s conclusions and working assumptions that will guide treatment of the patient.
Information about a person who participates or is expected to participate in the care of a patient.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Non-imaging and non-laboratory tests performed that result in structured or unstructured findings specific to the patient to facilitate the diagnosis and management of conditions.
Tests that result in visual images requiring interpretation by a credentialed professional.
Information related to interactions between healthcare providers and a patient.
Desired state to be achieved by a patient.
Data related to an individual’s insurance coverage for health care.
Assessments of a health-related matter of interest, importance, or worry to a patient, patient’s family, or patient’s healthcare provider that could identify a need, problem, or condition.
Record of vaccine administration.
Analysis of clinical specimens to obtain information about the health of a patient.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Data used to categorize individuals for identification, records matching, and other purposes.
Condition, diagnosis, or reason for seeking medical attention.
Activity performed for or on a patient as part of the provision of care.
The metadata, or extra information about data, regarding who created the data and when it was created.
Unique identifier(s) for a patient's implantable device(s).
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
USCDI V3.1
Please read the USCDI v3.1 standard document and the ONC Standards Bulletin 22-2 for details. USCDI version 3.1 updates USCDI version 3 with the following changes: consistent with Executive Order 14168, the Sex, Sexual Orientation, and Gender Identity data elements have been removed or updated in the Patient Demographics/Information Data Class.
Harmful or undesired physiological responses associated with exposure to a substance.
Health professional’s conclusions and working assumptions that will guide treatment of the patient.
Information about a person who participates or is expected to participate in the care of a patient.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Non-imaging and non-laboratory tests performed that result in structured or unstructured findings specific to the patient to facilitate the diagnosis and management of conditions.
Tests that result in visual images requiring interpretation by a credentialed professional.
Information related to interactions between healthcare providers and a patient.
Desired state to be achieved by a patient.
Data related to an individual’s insurance coverage for health care.
Assessments of a health-related matter of interest, importance, or worry to a patient, patient’s family, or patient’s healthcare provider that could identify a need, problem, or condition.
Record of vaccine administration.
Analysis of clinical specimens to obtain information about the health of a patient.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Data used to categorize individuals for identification, records matching, and other purposes.
Condition, diagnosis, or reason for seeking medical attention.
Activity performed for or on a patient as part of the provision of care.
The metadata, or extra information about data, regarding who created the data and when it was created.
Unique identifier(s) for a patient's implantable device(s).
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
USCDI V4
USCDI v4 added 20 data elements and one data class to USCDI v3. Please reference the USCDI v4 standard document and the ONC Standards Bulletin 23-2 for details. To review the prioritization criteria ONC used to select the USCDI v4 data elements, refer to the ONC Standards Bulletin 22-2.
Harmful or undesired physiological responses associated with exposure to a substance.
Information about a person who participates or is expected to participate in the care of a patient.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Non-imaging and non-laboratory tests performed that result in structured or unstructured findings specific to the patient to facilitate the diagnosis and management of conditions.
Tests that result in visual images requiring interpretation by a credentialed professional.
Information related to interactions between healthcare providers and a patient.
Physical place of available services or resources.
Desired state to be achieved by a person or a person’s elections to guide care.
Data related to an individual’s insurance coverage for health care.
Assessments of a health-related matter of interest, importance, or worry to a patient, patient’s family, or patient’s healthcare provider that could identify a need, problem, or condition.
Record of vaccine administration.
Analysis of clinical specimens to obtain information about the health of a patient.
An instrument, machine, appliance, implant, software or other article intended to be used for a medical purpose.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Data used to categorize individuals for identification, records matching, and other purposes.
Information that guides treatment of the patient and recommendations for future treatment.
Condition, diagnosis, or reason for seeking medical attention.
Activity performed for or on a patient as part of the provision of care.
The metadata, or extra information about data, regarding who created the data and when it was created.
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
USCDI V5
USCDI v5 was published on July 16, 2024, and includes 16 new data elements and two new data classes. Please read the USCDI v5 standard document and the ONC Standards Bulletin 24-2 for details.
Harmful or undesired physiological responses associated with exposure to a substance.
Information about a person who participates or is expected to participate in the care of a patient.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Non-imaging and non-laboratory tests performed that result in structured or unstructured findings specific to the patient to facilitate the diagnosis and management of conditions.
Tests that result in visual images requiring interpretation by a credentialed professional.
Information related to interactions between healthcare providers and a patient.
Physical place of available services or resources.
Desired state to be achieved by a person or a person’s elections to guide care.
Data related to an individual’s insurance coverage for health care.
Assessments of a health-related matter of interest, importance, or worry to a patient, patient’s family, or patient’s healthcare provider that could identify a need, problem, or condition.
Record of vaccine administration.
Analysis of clinical specimens to obtain information about the health of a patient.
An instrument, machine, appliance, implant, software or other article intended to be used for a medical purpose.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Findings or other clinical data collected about a patient during care.
Provider-authored request for the delivery of patient care services.
Usage notes: Orders convey a provider’s intent to have a service performed on or for a patient, or to give instructions on future care.
Data used to categorize individuals for identification, records matching, and other purposes.
Information that guides treatment of the patient and recommendations for future treatment.
Condition, diagnosis, or reason for seeking medical attention.
Activity performed for or on a patient as part of the provision of care.
The metadata, or extra information about data, regarding who created the data and when it was created.
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
USCDI V6
ASTP/ONC published USCDI v6 on July 24, 2025, which includes 6 new data elements. Please read the USCDI v6 Standard Document and the ASTP/ONC Standards Bulletin 25-2 for details. ASTP/ONC welcomes input on future versions during the USCDI feedback period, open through September 29, 2025, at 11:59 PM ET. During this time, ASTP/ONC is accepting new data element submissions through ONDEC, and comments on existing data elements may be entered via the updated commenting feature on the USCDI data element pages.
Harmful or undesired physiological responses associated with exposure to a substance.
Information that guides treatment of the patient and recommendations for future treatment.
Information about a person who participates or is expected to participate in the care of a patient.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Non-imaging and non-laboratory tests performed that result in structured or unstructured findings specific to the patient to facilitate the diagnosis and management of conditions.
Tests that result in visual images requiring interpretation by a credentialed professional.
Information related to interactions between healthcare providers and a patient.
Physical place of available services or resources.
Family member health condition(s) that are relevant to a patient's care.
Desired state to be achieved by a person or a person’s elections to guide care.
Data related to an individual’s insurance coverage for health care.
Assessments of a health-related matter of interest, importance, or worry to a patient, patient’s family, or patient’s healthcare provider that could identify a need, problem, or condition.
Record of vaccine administration.
Analysis of clinical specimens to obtain information about the health of a patient.
An instrument, machine, appliance, implant, software or other article intended to be used for a medical purpose.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Provider-authored request for the delivery of patient care services.
Usage notes: Orders convey a provider’s intent to have a service performed on or for a patient, or to give instructions on future care.
Data used to categorize individuals for identification, records matching, and other purposes.
Condition, diagnosis, or reason for seeking medical attention.
Activity performed for or on a patient as part of the provision of care.
The metadata, or extra information about data, regarding who created the data and when it was created.
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
- Represented by a terminology standard or SDO-balloted technical specification or implementation guide.
- Data element is captured, stored, or accessed in multiple production EHRs or other HIT modules from more than one developer.
- Data element is electronically exchanged between more than two production EHRs or other HIT modules of different developers using available interoperability standards.
- Use cases apply to most care settings or specialties.
Level 2
Harmful or undesired physiological responses associated with exposure to a substance.
Material substance originating from a biological entity intended to be transplanted or infused into another (possibly the same) biological entity.
Tests that result in visual images requiring interpretation by a credentialed professional.
Physical place of available services or resources.
Data related to an individual’s insurance coverage for health care.
Record of vaccine administration.
An instrument, machine, appliance, implant, software or other article intended to be used for a medical purpose.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Provider-authored request for the delivery of patient care services.
Usage notes: Orders convey a provider’s intent to have a service performed on or for a patient, or to give instructions on future care.
Data used to categorize individuals for identification, records matching, and other purposes.
Activity performed for or on a patient as part of the provision of care.
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
- Represented by a terminology standard or SDO-balloted technical specification or implementation guide.
- Data element is captured, stored, or accessed in at least one production EHR or HIT module.
- Data element is electronically exchanged between two production EHRs or other HIT modules using available interoperability standards.
- Use cases apply to several care settings or specialties.
Level 1
Material substance originating from a biological entity intended to be transplanted or infused into another (possibly the same) biological entity.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Physical place of available services or resources.
Assessments of a health-related matter of interest, importance, or worry to a patient, patient’s family, or patient’s healthcare provider that could identify a need, problem, or condition.
Analysis of clinical specimens to obtain information about the health of a patient.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Data used to categorize individuals for identification, records matching, and other purposes.
The metadata, or extra information about data, regarding who created the data and when it was created.
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.
- Not represented by a terminology standard or SDO-balloted technical specification or implementation guide.
- Data element is captured, stored, or accessed in limited settings such as a pilot or proof of concept demonstration.
- Data element is electronically exchanged in limited environments, such as connectathons or pilots.
- Use cases apply to a limited number of care settings or specialties, or data element represents a specialization of other, more general data elements.
Level 0
Harmful or undesired physiological responses associated with exposure to a substance.
Material substance originating from a biological entity intended to be transplanted or infused into another (possibly the same) biological entity.
Information about a person who participates or is expected to participate in the care of a patient.
Narrative patient data relevant to the context identified by note types.
-
- Usage note: Clinical Notes data elements are content exchange standard agnostic. They should not be interpreted or associated with the structured document templates that may share the same name.
Non-imaging and non-laboratory tests performed that result in structured or unstructured findings specific to the patient to facilitate the diagnosis and management of conditions.
Tests that result in visual images requiring interpretation by a credentialed professional.
Information related to interactions between healthcare providers and a patient.
Health data as reflected in a patient's Explanation of Benefits (EOB) statements, typically derived from claims and other administrative data.
Desired state to be achieved by a patient.
Desired state to be achieved by a person or a person’s elections to guide care.
Data related to an individual’s insurance coverage for health care.
Assessments of a health-related matter of interest, importance, or worry to a patient, patient’s family, or patient’s healthcare provider that could identify a need, problem, or condition.
Record of vaccine administration.
Analysis of clinical specimens to obtain information about the health of a patient.
An instrument, machine, appliance, implant, software or other article intended to be used for a medical purpose.
Pharmacologic agents used in the diagnosis, cure, mitigation, treatment, or prevention of disease.
Findings or other clinical data collected about a patient during care.
Provider-authored request for the delivery of patient care services.
Usage notes: Orders convey a provider’s intent to have a service performed on or for a patient, or to give instructions on future care.
Data used to categorize individuals for identification, records matching, and other purposes.
Information that guides treatment of the patient and recommendations for future treatment.
Condition, diagnosis, or reason for seeking medical attention.
Activity performed for or on a patient as part of the provision of care.
The metadata, or extra information about data, regarding who created the data and when it was created.
Physiologic measurements of a patient that indicate the status of the body’s life sustaining functions.




The USCDI ONC New Data Element and Class (ONDEC) Submission System supports a predictable, transparent, and collaborative process, allowing health IT stakeholders to submit new data elements and classes for future versions of USCDI. Click here for more information and to submit new data elements.
The USCDI standard will follow the Standards Version Advancement Process described in the Cures rule to allow health IT developers to update their systems to newer version of USCDI and provide these updates to their customers.
Comment
Submitted by melanie.kourba… on
Assigning Authority
APHL recommends ASTP include the assigning authority and identifier code typewith ANY identifier data element (in all HL7 products, assignment authority is part of the various supported identifier type data types). This comment applies to Identifier (Identifier), Specimen Identifier (Specimen Identifier), Medical Record Number (Medical Record Number), and Medicare Patient Identifier (Medicare Patient Identifier). We recommend that the definitions for these data elements include the following language: "Alphanumeric value that uniquely identifies the declared identifier type over time, at minimum within one organization, ideally at the national level, including a means to identify the organization or system that assigned it."
Ideally, the complete identifier should consist of the alphanumeric string, the assigning authority, and the identifier code type. This combination would promote data interoperability by allowing data modelers to confidently merge alphanumeric strings that are identical. Just because the alphanumeric string in the Identifier field in two different messages is identical, the data modeler cannot assume that the patient is the same. It may be that the string refers to a medical record number in one and a patient identifier in another, or to medical record numbers from two different EHR implementation that just happen to be the same. A more complete identifier with assigning authority and identifier code type would eliminate this confusion and lead to more transparent, interoperable data and ultimately actionable data.
Submitted by jkegerize on
ACLA General USCDI Comment
The American Clinical Laboratory Association (ACLA) recommends that while FHIR may be used for these standards, the continued use of other formats—such as HL7 v2.x and CDA—should also be permitted, given their widespread adoption in existing applications.
Submitted by Robert Hussey on
General Comments on USCDI v7
Attached please find comments from Wolters Kluwer on Version 7 of the United States Core Data for Interoperability. Thanks for the opportunity to share our views.
Submitted by mashafa on
Academy of Nutrition and Dietetics feedback for USCDI v7
The Academy of Nutrition and Dietetics appreciates the opportunity to provide input on the United States Core Data for Interoperability (USCDI) Version 7. Please find our detailed comments attached for your review and consideration.
Submitted by NCQA on
NCQA is pleased to provide the following recommendations, summarized below and detailed on the Data Class ONDEC pages for USCDI v7.
We recommend modifications to the following USCDI elements:
1. Health Status Assessments, Smoking Status: Expand the element definition to include tobacco use and expand the terminology to include LOINC.
2. Diagnostic Imaging, Diagnostic Imaging Report: Expand the terminology requirements for structured capture of interpretation of results (SNOMED, RadLex).
3. Clinical Notes, Discharge Summary Note: Revise the required elements of discharge summaries to align to practice.
4. Patient Information, Race and Ethnicity: Update the Race and Ethnicity data elements to align with the OMB revisions to the SPD 15, published on March 2024.
We recommend adding the following elements to USCDI v7:
1. Orders, Referral Orders
2. Orders, Medical Device Orders
3. Health Status Assessments, Goal Assessment (new submission)
4. Explanation of Benefits, Carin Blue Button CPCDS elements
Submitted by jaylyle on
Guidance for standards developers
On behalf of the Department of Veterans Affairs
We on the HL7 C-CDA-to-FHIR Mapping project applaud the work USCDI has done to establish a baseline of record data that providers and users of the data can rely on, irrespective of the data specification used to transmit it.
We believe that it is necessary for the specifications to be commensurable. I.e., a clinician viewing allergies on a screen from two data sources, one CDA and one FHIR, should see them presented in a consistent manner, and the CDS, quality measure, and other automated systems that consume this information should likewise be able to make unambiguous and warranted assumptions about equivalences. Many existing systems perform this kind of translation, and in most cases it is straightforward, but there are many cases where independent stakeholders may make divergent assumptions. These divergences are likely to create safety risks. We believe that the translations must be informed by collective understanding that is consistent and expressed in a computable manner.
Some of these translations may be lossy, but they all need to be clear and explicit, whether lossy or not. A clear understanding of the gaps is as important as the lossless maps.
We would like to see ASTP direct the creators of regulation-identified specifications based on USCDI requirements (viz., C-CDA & FHIR US Core) to agree on and document translations.
Submitted by fiehnr on
ADA Comments on USCDI
The American Dental Association applauds the ASTP/ONC leadership in advancing interoperability, and continued efforts to expand and refine the USCDI as a foundation for nationwide health information exchange. We appreciate the opportunity to submit comments on the proposed United States Core Data for Interoperability (USCDI) Version 7.
Submitted by Xueting on
Support for New Data Elements in Draft USCDI v6
As a developer of a wearable-based health platform focused on preventive, patient-centered care, I strongly support several of the newly introduced data elements in Draft USCDI v6. These additions represent a meaningful step toward enabling next-generation digital health solutions that are already technically ready and in active use.
- Unique Device Identifier (UDI):
Expanding this field to include non-implantable devices is a critical and necessary update. Many high-utility wearable devices—such as smart rings, biosensing earbuds, and connected watches—require structured identification for traceability, recall safety, and post-market surveillance. This change directly supports real-world device integration into the national data framework. - Care Plan:
This element goes far beyond traditional provider-authored orders. It enables personalized, multi-party care planning that includes patients, families, and digital tools. For platforms like ours, which generate dynamic feedback and goal-tracking based on real-time biometrics, the Care Plan provides a structured path for cross-setting collaboration without requiring prescriptive authority. - Family Health History:
A crucial field for preventive care, this data element supports 2C digital platforms in personalizing risk stratification, early screening recommendations, and long-term health trajectory modeling. Its inclusion validates many consumer-directed innovations in genomics and women's health. - Date of Onset:
This is one of the most valuable additions to USCDI v6. It enables precise disease timeline modeling, differential diagnosis, and the detection of subtle, progressive patterns in chronic or behavioral conditions—especially when powered by continuous wearable monitoring. - Facility Address:
This field supports the future of connected care through geospatial analysis and IoT integration. It will allow real-time mapping of care capacity, patient flow, and even environmental health risks. For wearable devices that track patient location or care site interaction, this field adds meaningful context.
Together, these fields represent a much-needed expansion of the USCDI model to reflect where healthcare is going: toward real-time, distributed, patient-initiated care models. We strongly encourage ONC to retain and prioritize these additions in the final version of USCDI v6.
Thank you for your leadership in modernizing U.S. health interoperability.
Sincerely,
Wearable Health Platform Developer
Submitted by Riki Merrick on
SHIELD Community Comment on…
SHIELD Community Comment on USCDI V6 Draft:
- SHIELD appreciates the role that USCDI plays in standardizing health data for interoperability in the laboratory space. However, the high level of the USCDI guidance leads to inconsistencies across the industry when individual health and laboratory entities need to determine individually how to fill in the gaps. The lack of a coherent data model is still a barrier to end to end laboratory interoperability and many of the SHIELD participants have noted that without a complete set of laboratory data, they are unable to validate and therefore integrate the laboratory data that is currently flowing into care processes and analytics, resulting in low-yield, low quality data exchanges.
SHIELD strongly encourages ASTP/ONC to consider expanding USCDI’s laboratory data class to more formally define a data model for the current collection of data elements, including more formal vocabulary bindings. This would add great value by improving laboratory data interoperability and could be an opportunity to onboard Laboratory Information Systems into FHIR data exchange with standardized source data. SHIELD would be excited to offer its industry, informatics and healthcare expertise to any effort to build out a shared laboratory data model with other laboratory interoperability stakeholders.
- In principle SHIELD supports the creation of single construct across classes in USCDI because it is an opportunity to improve data across healthcare.
- SHIELD suggests to focus on the device model part of the UDI, as that may be sufficient and easier to ingest into the documentation flow to start.
- In the laboratory domain being able to include the device identifier (we previously had requested inclusion of USCDI Data Element Test Kit Unique Identifier and USCDI Data Element Instrument Unique Indentifier) which is critical for reconcilliation for effective data artifacts deduplication, for example as part of clinical trials, in patient charts for laboratory results from different institutions and for creation of Real World Evidence (RWE). Without sufficient details about the performed test, EHR-systems cannot safely ingest data, utilize in chart views, which may impact patient outcomes. Laboratory data occupies 90% of clinical records and laboratory results support 80% of diagnosis. Here we reiterate the requests to include USCDI Data Element Test Kit Unique Identifier and USCDI Data Element Instrument Unique Identifier in USCDIv6.
HIT should support the addition of UDIs to laboratory results, the updating of UDIs (if entered in error), the association of one or more UDIs to a laboratory result, exchange of the UDI with laboratory results, receipt of all UDIs associated to laboratory results and search for UDIs and/or laboratory results reflecting both.
- SHIELD suggests ASTP/ONC clarify the scope of this data element as the current definition is not clear enough:
- Given that ASTP/ONC associates LOINC as the required vocabulary for Laboratory Orders, most likely the data element targeted here is “Ordered Test” which should cover both the code (drawn from LOINC and available as USCDI+ Public Health Lab Use Case Laboratory Test/Panel Code as well as the display name for that test in the Laboratory Compendium (CLIA requirement) compared to the CLIA defintion of what the test request needs to include (42 CFR 493.1241 -- Standard: Test request. ).
- SHIELD supports the use of LOINC when an appropriate code is available for Laboratory Orders whenever they are being communicated externally and should cover all orders; the initial order, individual occurrences of a multi-occurrence order, individual tests from a larger panel ordered to a reference laboratory, reflex, etc.
Performance Time (under Procedure):
- Since laboratory tests are not medical procedures performed on a patient, SHIELD suggests ASTP/ONC consider adding the following widely implemented (through HL7 v2 laboratory result messaging in support of CMS CLIA regulations) laboratory test related date/time stamps to V6:
- as equivalent to procedure performance time: test result date/time (Definition: when the analysis is performed on the specimen(s) creating the observation, or when the result is calculated on existing observations.) We would argue that this is a Level 2 element already because of its widespread capture and use in healthcare.
- to track and understand when results were available outside the laboratory as well as to understand any updates to laboratory results over time: reporting date/time (Definition: when the results are verified and released for reporting.; the requested laboratory reporting equivalent to PH / Case Reporting / Date of the Report). We would argue that this is a Level 2 element already because of its widespread capture and use in healthcare.
- clinically important for interpretation of results: Laboratory / Specimen collection date/time / Level 0: (Definition: Date/time when clinical specimen was collected from subject patient. This is the clinically relevant date time of an observation when a specimen is collected for a test.) We would argue that this is a Level 2 element already because of its widespread capture and use in healthcare.
- The proposed elements should not be considered Level 0 or 1; they must at least be elevated to Level 2 given their wide adoption and availability and the fact that they are required under CMS CLIA regulations.
SHIELD suggests use of UCUM for unit in measure unless the test has an element that is not captured through a standardized set of units (such as a clinical set of criteria specific to an individual laboratory test).
Updated vocabulary:
- SHIELD supports the use of LOINC when an appropriate code is available for Laboratory Orders whenever they are being communicated externally and should should cover all orders; the initial order, individual occurrences of a multi-occurrence order, individual tests from a larger panel ordered to a reference laboratory, reflex, etc.
- SHIELD suggests to update to the latest available version at time of publication of V6 for all called out coding systems/terminologies.
- SHIELD suggests ASTP/ONC clarify the scope of this data element as the current definition is not clear enough:


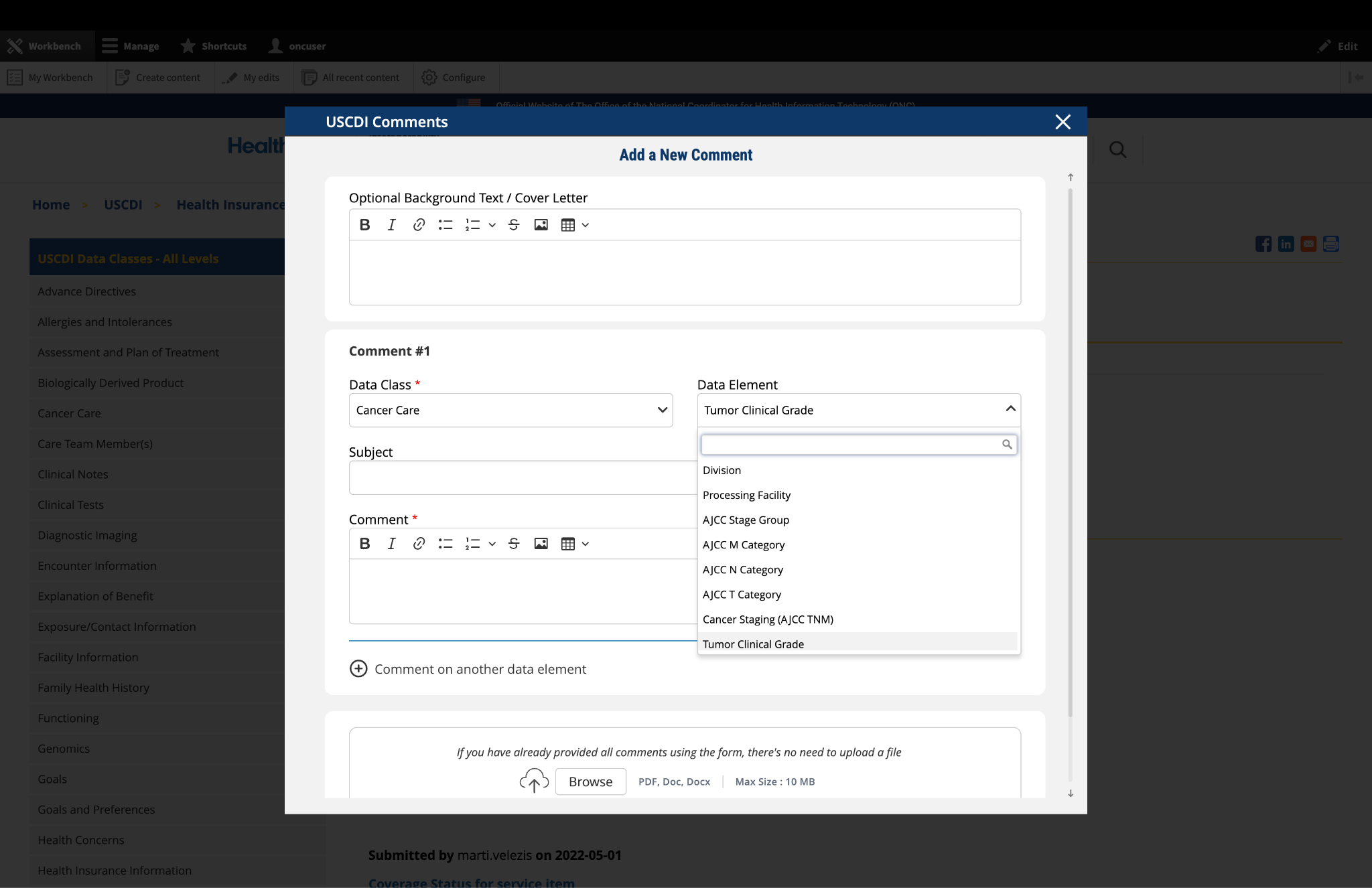
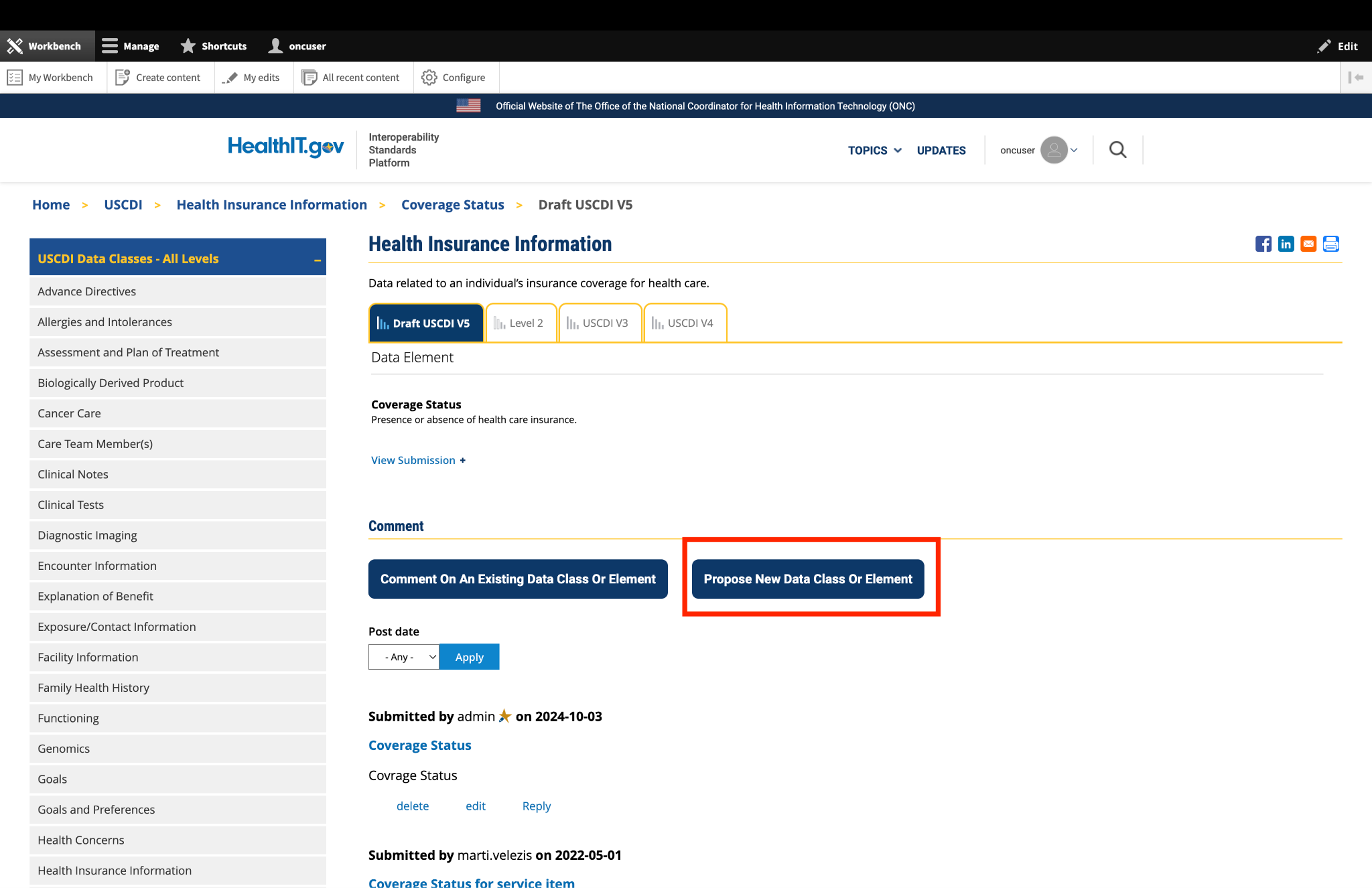
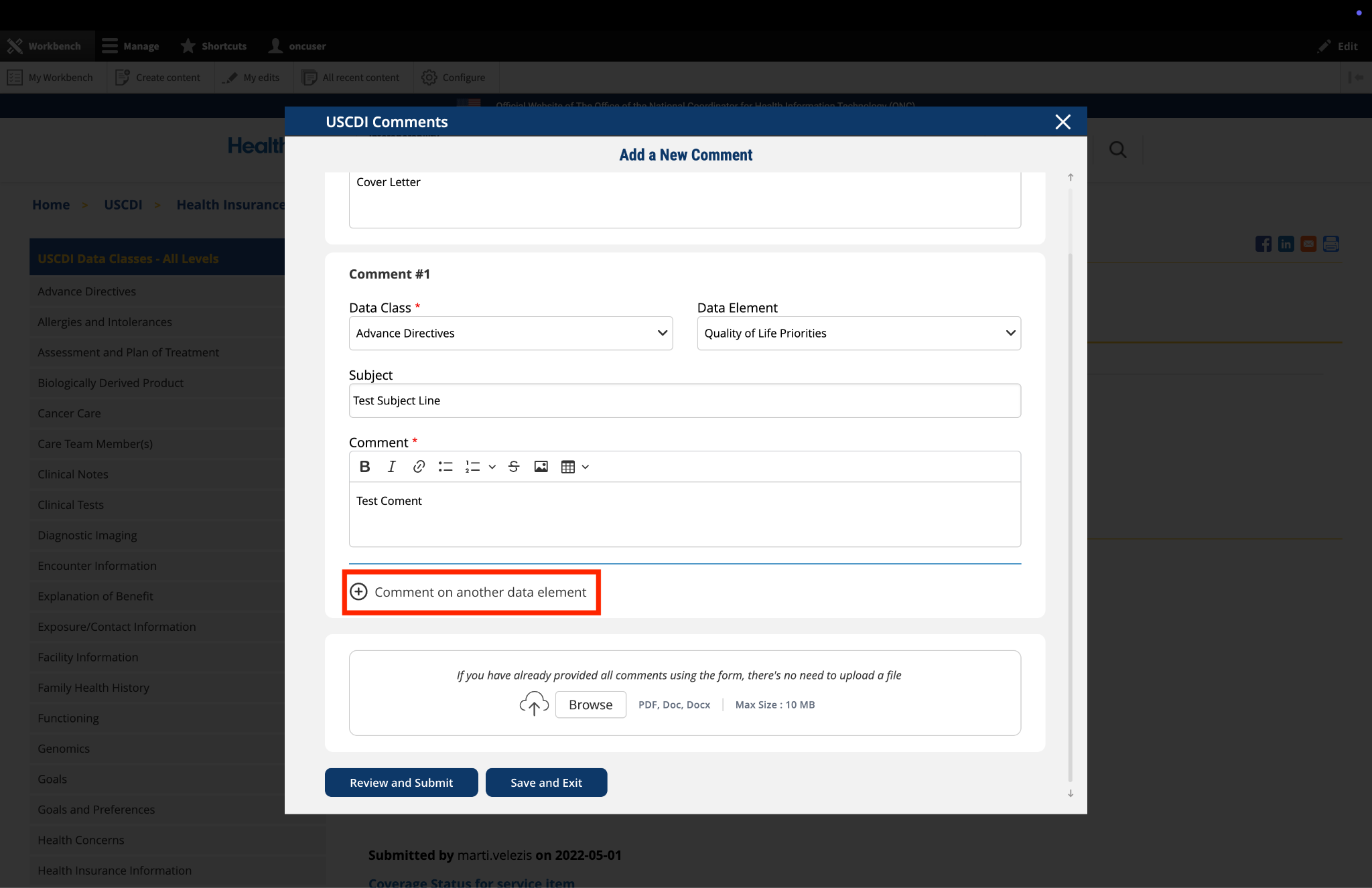
Submitted by Parul.goyal on
Becton, Dickinson and Company (BD) comments on USCDI Version 7
BD (Becton, Dickinson and Company) commends the Offices of the Assistant Secretary for Technology Policy (ASTP) and the National Coordinator for Health Information Technology (ONC) for their leadership in advancing data interoperability and digital advancements in healthcare. We support the administration’s broader innovation agenda and welcome the opportunity to contribute to this important work.
BD is committed to ensuring patients have access to medical technologies globally, touching billions of patients and helping improve and save lives. In the U.S., BD manufactures more medical devices than any other company and operates more than 30 manufacturing sites and distribution centers across the country. Throughout our 128-year history, BD has been a long-standing partner to the U.S. government in its efforts to ensure access to innovative medical technologies that are critical to American healthcare. Our focus on interoperability and digital quality measures reflects our dedication to reducing harm and supporting frontline care teams.
We recognize the importance of the USCDI framework in building scalable, standardized systems that enhance care and safety. As updates are considered, we offer the following recommendations:
We recognize this is an iterative process and look forward to providing detailed input in future comment cycles. Please don’t hesitate to reach out to us with any questions in the meantime.
Jessica C. Johnston
Vice President, Market Access and Payment Policy