Submitted by nedragarrett_CDC on
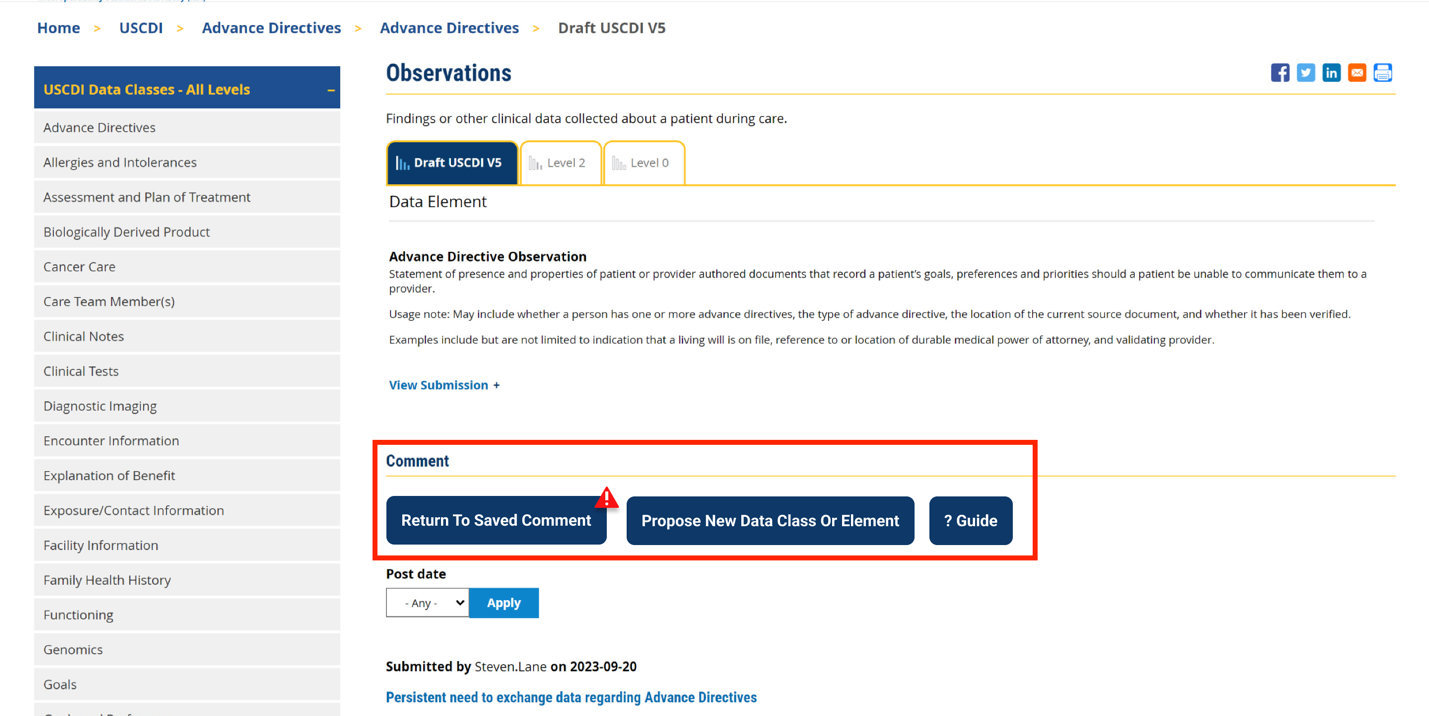
CDC's Comment for draft USCDI v6
The integration of the "Medical Record Number" as a standardized data element in USCDI v6 is essential for bolstering public health efforts, particularly in the realm of immunization. As a unique identifier, it ensures that vaccination records are accurately matched to individual patients, which is crucial for effective immunization programs.
Standardizing the Medical Record Number enhances the ability of public health authorities to monitor immunization coverage, assess vaccine effectiveness, and identify pockets of under-vaccinated populations. This capability is especially important for coordinating responses to vaccine-preventable disease outbreaks and managing mass vaccination campaigns during pandemics.
Furthermore, precise patient matching enabled by a consistent Medical Record Number helps prevent duplicate immunization records and ensures that individuals receive vaccines according to recommended schedules. It also facilitates accurate reporting to immunization information systems (IIS), supporting real-time public health decision-making and resource allocation.
Inclusion of the "Medical Record Number" in USCDI v6 promises to significantly improve the accuracy of patient identification for immunizations, thereby enhancing public health surveillance capabilities and ensuring successful implementation of national immunization strategies. Urgent consideration for this inclusion is recommended to strengthen overall public health initiatives.


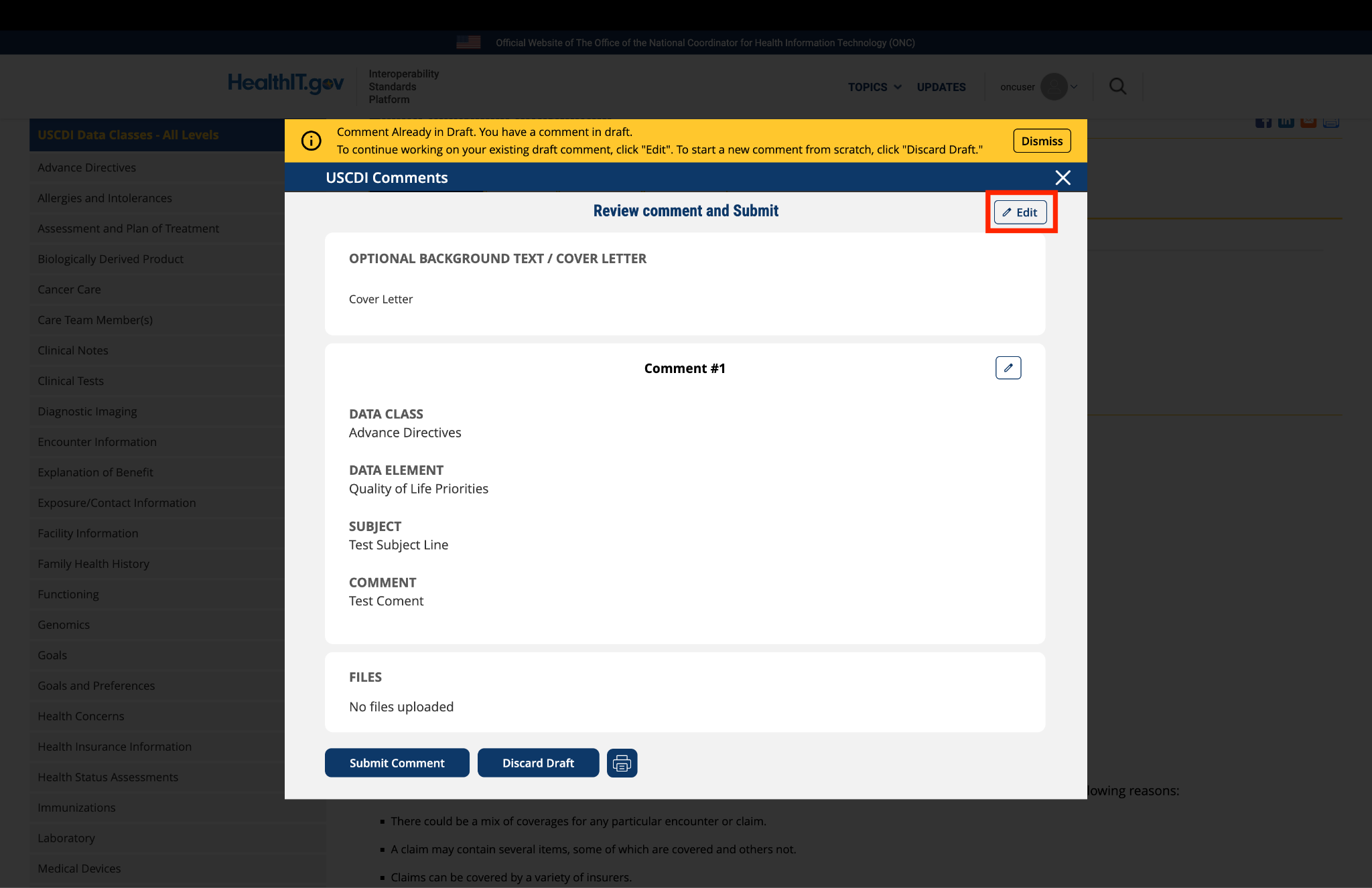
Submitted by nedragarrett_CDC on
CDC's comment for proposed inclusion in USCDI v7
National Hospital Care Survey (NHCS) and National Ambulatory Medical Care Survey (NAMCS) both conduct data linkages to other datasets (e.g. National Death Index, the U.S. Department of Housing and Urban Development, etc.) and publish them as restricted use data files. This data element will increase the ability to track unique patients, improving accuracy and quality in these produced datasets.
Location of data element in the current HCS CDA IG V1.2: x-path /ClinicalDocument/recordTarget/patientRole/patient/id)
This data element is in C-CDA 4.0. Link in the C-CDA IG- https://hl7.org/cda/us/ccda/StructureDefinition-USRealmHeader.html
Description of what DHCS currently receives in production: All EHR files submitted to NHCS lack medical record number (MRN).