About
ONC welcomes from any entity (including health IT developers, standard development organizations and other consortia) the submission of test procedures, test tools and associated test data (herein a "test method") for consideration for approval and use under the ONC Health IT Certification Program (Certification Program). ONC’s goal is to optimize the certification experience by expanding its comprehensive portfolio of testing technology to support and validate the criteria and related requirements of the Certification Program. A diverse mix of testing tools, including those developed in partnership with or solely administered by industry, or those transferred from government to industry can help optimize the certification experience.
Background
On January 7, 2011, the Department of Health and Human Services(HHS) issued a final rule establishing a permanent certification program for the purposes of testing and certifying health information technology (“Establishment of the Permanent Certification Program for Health Information Technology,” 76 FR 1262) (“Permanent Certification Program final rule”). The program has since been renamed to the “ONC Health IT Certification Program” (80 FR 16804, 16806). The Permanent Certification Program final rule outlines provisions for a person or entity to submit a test procedure or test tool including any associated test data to the National Coordinator for Health Information Technology to be considered for approval and use.
On June 9, 2015 HHS published a notice in the Federal Register titled "Acceptance and Approval of Non-Governmental Developed Test Procedures, Test Tools, and Test Data for Use under the ONC Health IT Certification Program." This notice and a related blog, informed the public of ONC’s policy that permits any person or entity to submit test method to ONC to be considered for approval and subsequent use by the ONC-Authorized Testing Laboratories (ONC-ATLs).
In August 2017, ONC published a blog post that discussed our transition to diversify the Certification Program’s testing. With the implementation of Title IV of the 21st Century Cures Act (Cures Act), ONC aims to transition the Certification Program’s existing testing portfolio over the next five years to include as many industry-developed and maintained testing tools as possible in lieu of tax-payer financed testing tools. Achieving this goal will enable the Certification Program to more efficiently focus its testing resources and better align with industry-developed testing tools, which could help support the “real world testing” envisioned by the Cures Act.
Application Process for Consideration of ONC-Approved Test Method
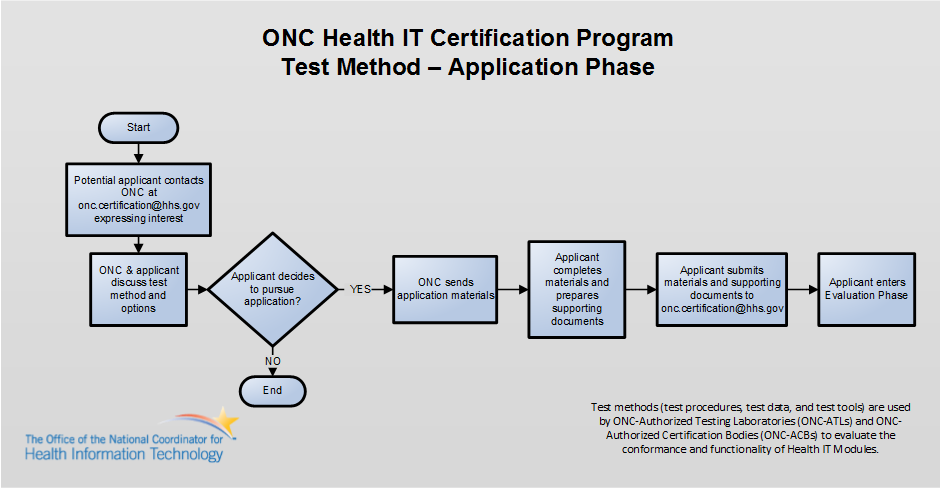
Entities interested in submitting an alternative test method for ONC’s consideration should first express interest to ONC via e-mail to ONC.Certification@hhs.gov to initiate the application phase. The diagram below illustrates the application phase.
Evaluation Process of Test Method
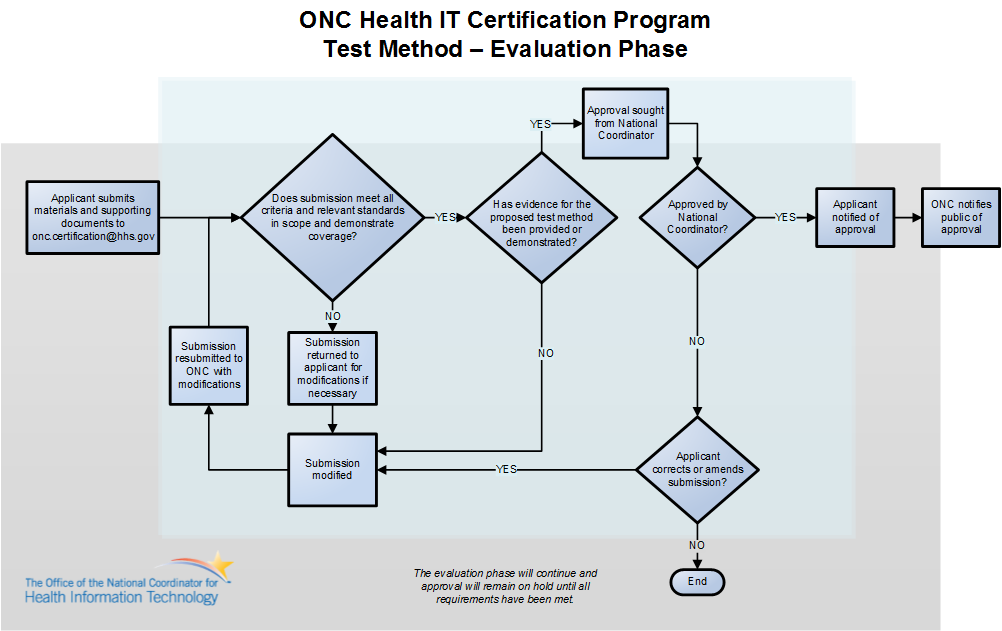
Alternative Test Method submissions will be reviewed by ONC for comprehensiveness and alignment with the Certification Program's requirements. The applicant may be required to demonstrate and/or provide documentation of the following during the evaluation process:
- The developer of the test method;
- Traceability to the applicable certification criterion or criteria, including which specific criteria are addressed by the test method and an explanation of how the test method would evaluate a Health IT Module’s compliance with the applicable criteria; and
- The process used to develop the test method, including any opportunity for the public to comment and the degree to which public comments were considered.
ONC reserves the right to require additional demonstration of and/or documentation supporting test method design, capacity, and functionalities as needed. The diagram below outlines the steps in the evaluation phase.
Approval and Implementation
Upon approval by the national coordinator, the applicant will be notified of the approval, and ONC will notify the public. Entities with an ONC-Approved Test Method will enter into a Memorandum of Understanding (MOU) with ONC to ensure continued alignment and support of the approved test method.
The complete set of Test Methods for the ONC Health IT Certification Program can be accessed here: Testing Process and Test Methods