The graphic below shows the organizational structure of the ONC Health IT Certification Program. As illustrated, ONC manages the overall program while working with other agencies and entities in the following capacities:
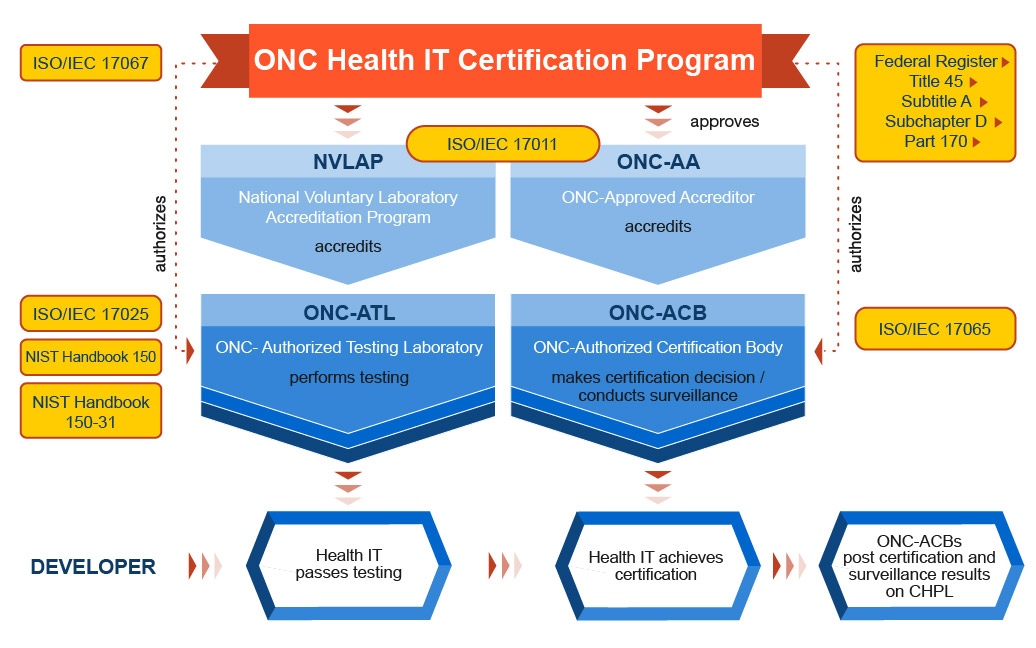
The ONC-Approved Accreditor (ONC-AA) accredits and oversees certification bodies. Accredited certification bodies must seek authorization from ONC to participate in the ONC Health IT Certification Program - once authorized, they are called ONC-Authorized Certification Bodies (ONC-ACBs).
NVLAP is the accreditor for testing laboratories in the ONC Health IT Certification Program. NVLAP accredited testing laboratories must seek authorization from ONC to test products under the ONC Health IT Certification Program. Once authorized, they are called ONC-Authorized Testing Laboratories. (ONC-ATLs A single organization can serve as both an ONC-ACB and an ONC-ATL, as long as a firewall is established between testing and certification activities.)
Developers or Vendors have their Health IT Module(s) tested by an ONC-ATL. After the Health IT Module has been successfully tested, it can be certified by an ONC-ACB. Products certified by an ONC-ACB are posted to the Certified Health IT Product List (CHPL).

