
An official website of the United States government
Here’s how you know
Official websites use .gov
A
.gov
website belongs to an official government organization in the
United States.
Secure .gov websites use HTTPS
A
lock
(
) or
https://
means you’ve safely connected to the .gov website. Share sensitive
information only on official, secure websites.
Standards Adoption Among Health Information Exchange Organizations
No. 75 | October 2024
Health information technology standards are used in the health care industry to facilitate the capture and exchange of information.1 Health information exchange organizations (HIOs) are state and regional networks that enable electronic exchange of health information across their participants, which may include health care providers, public health agencies, payers, and other health care entities. HIOs’ adoption and conformance to standards is critical to enable the seamless sharing of information among their participants and across networks to ensure that information can be easily integrated once received.2 Understanding how HIOs use standards can inform public and private efforts to promote better coordination on standards development, implementation, and conformance. As there are various standards in use across health IT for storing and transporting data, we surveyed HIOs nationwide to assess current adoption and use of various standards, including the United States Core Data for Interoperability (USCDI) and Health Level 7 (HL7®) Fast Healthcare Interoperability Resources (FHIR®). This brief reports on the share of HIOs in 2023 that electronically send and receive data using these standards, and the range of data elements they make available to network members.
Highlights
- A majority of HIOs routinely sent and received HL7 Version 3 Clinical Document Architecture (CDA) documents and HL7v2 messages.
- On average, HIOs made more than two-thirds of data elements from USCDI versions 1-4 available to participating organizations.
- HIOs were more likely to send data (or make data available) that adhere to USCDI v1 or v2, but less likely to receive data from participants that adhere to USCDI v1 or v2.
- Nearly half (46%) of HIOs mapped from non-standard laboratory test or result codes to LOINC codes when accessing data from labs.
- Over half of HIOs that have mapped from non-standard laboratory test or result codes to LOINC codes reported they have not experienced any issues with LOINC mapping.
A majority of HIOs routinely sent and received CDA documents and HL7v2 messages.
Findings
- More than 90% of HIOs reported that they routinely or sometimes sent or made available to and received CDA documents from their participants.
- More than 80% of HIOs reported that they routinely or sometimes sent (or made available) and received any type of HL7 v2 messages.
- Ninety percent of HIOs routinely received data from their participants in the format of HL7 v2 admission, discharge, and transfer (ADT) messages.
- About one-fifth of HIOs routinely or sometimes sent or made data available and received data via HL7 FHIR APIs.
Figure 1: Percentage of HIOs that reported routinely or sometimes sending or making data available and receiving data from their participants, by approach.

Notes: N=68 for send/make data available, 9 missing responses were excluded from the denominator. N=63 for receive, 14 missing responses were excluded from the denominator. “HL7 FHIR APIs” include respondents who selected HL7 FHIR messages (DSTU2), HL7 FHIR Release 3 (STU) messages, or FHIR v.4.0 messages. See Appendix Table A1 for survey questions and Appendix Tables A2 and A3 for more details.
HIOs, on average, reported making more than two-thirds of data elements included* in the USCDI standard available to participating organizations.
Findings
- Based on an analysis of data elements made available by HIOs (see Appendix Table A4) to participating organizations and included in USCDI versions 1-4, HIOs on average made available 73% of those data elements included in USCDI v1, 72% of those data elements included in v2, and 67% of those data elements included in versions 3-4.
- Among all data elements included in the survey, HIOs made available immunizations (92%), problems (90%), vital signs (90%), and encounters (90%) data elements to participating organizations at the highest rates.
- Among all data elements included in the survey, HIOs made available, substance use disorder (39%), diagnostic imaging order (51%), and care plan (51%) data elements to participating organizations at the lowest rates.
Table 1: Data elements made available by HIOs and included in USCDI versions 1-4, ranked by frequency, and the average rate of data element availability by USCDI version.
| Data Element | USCDI Versions | % of HIOs |
|---|---|---|
| Most Frequently Made Available (Top 4) | ||
| Immunizations | 1, 2, 3, 4 | 92% |
| Problems | 1, 2, 3, 4 | 90% |
| Vital Signs | 1, 2, 3, 4 | 90% |
| Prescribed Medications | 1, 2, 3, 4 | 87% |
| Least Frequently Made Available (Bottom 3) | ||
| Substance Use Disorder | 4 | 39% |
| Diagnostic Imaging Order | — | 51% |
| Care Plan Fields | 1, 2, 3, 4 | 51% |
| USCDI Version(s) | Average Rate of Availability of Data Elements | |
| Data elements included in version 1 | 73% | |
| Data elements included in version 2 | 72% | |
| Data elements included inversion 3/4 | 67% | |
| Data elements included in versions 1-4 | 67% | |
Notes: This table reflects an analysis of data elements asked about in survey question Q4 mapped to USCDI versions 1-4; this survey question does not include the full list of USCDI data elements. See Appendix Table A1 for question text and the Methods section for more details on the analysis/mapping and its interpretation. See Appendix Table A4 for a full list of data elements asked by the survey, the USCDI versions these data elements are included in, and the proportion of HIOs that reported making each data element available to participating organizations.
One in three HIOs routinely received data that adhere to USCDI v1 or v2 from participants.
Findings
- Twenty-two percent of HIOs reported sometimes receiving data that adhere to USCDI v1 or v2 from participants from participants.
- Few HIOs reported rarely (11%) and never (2%) receiving data that adhere to USCDI v1 or v2 from participants.
- Thirty percent of HIOs responded that they don’t know if data received from participants adhere to USCDI v1 or v2.

Notes: N=63. 14 missing responses were excluded from the denominator. See Appendix Table A1 for survey questions. See Methods section for further details.
Forty-four percent of HIOs routinely sent or made data that adhere to USCDI v1 or v2 available to participants.
Findings
- Few HIOs reported sometimes (10%) and rarely (5%) sending or making data that adhere to USCDI v1 or v2 available to participants.
- Eight percent of HIOs reported never sending or making data available to participants that adhere to USCDI v1 or v2.
- Twenty-nine percent of HIOs reported that they don’t know if data sent or made available to participants adhered to USCDI v1 or v2.
Figure 3: Percentage of HIOs that reported sending or making data that adhere to USCDI v1 or v2 available to participants.
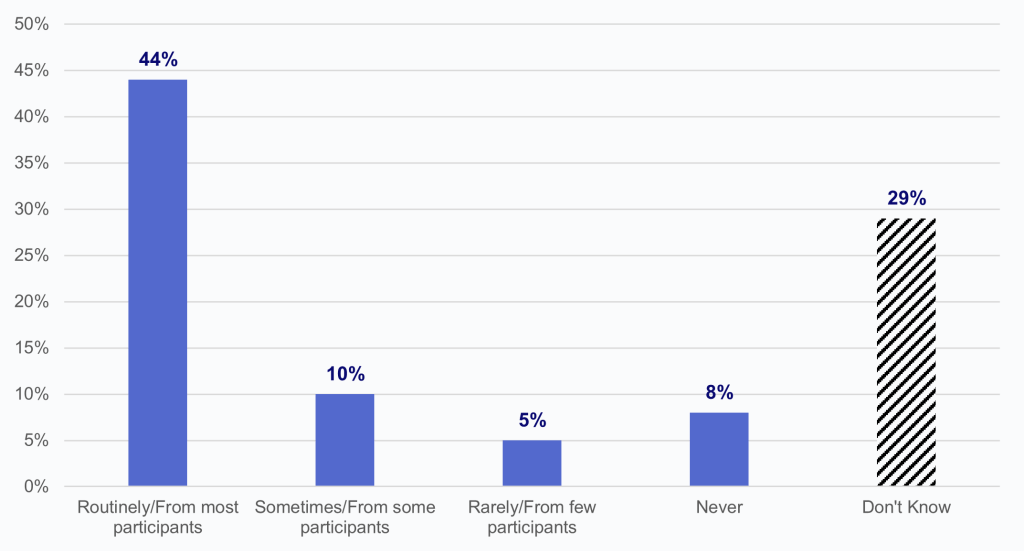
Notes: N=63. 14 missing responses were excluded from the denominator. See Appendix Table A1 for survey questions. See Methods section for further details.
Nearly half (46%) of HIOs map from non-standard laboratory test or result codes to LOINC codes.
Findings
- Forty-six percent of HIOs map from non-standard laboratory test or result codes to LOINC codes.
- Among HIOs that are mapping to LOINC codes, a majority reported having to map all or most (33%) or some (40%) non-standard laboratory test or result codes to LOINC codes.
- Among HIOs that are mapping to LOINC codes, 1 in 5 reported having to map few (17%) or no (3%) laboratory test or result codes from non-standard codes to LOINC codes.
Figure 4: Percentage of HIOs that map from non-standard laboratory test or results to LOINC codes and the extent to which HIOs reported having to map laboratory results.
Figure 4a. Does your HIE map from non-standard laboratory test/result codes to LOINC codes?
Figure 4b. If yes, within the past year, to what extent did your HIE have to map those results from non-standard codes to LOINC codes?

Notes: Figure 4a: N=65, 12 missing responses were excluded from the denominator. Figure 4b: N=29, 1 missing response was excluded from the denominator. See Appendix Table A1 for survey questions.
Among HIOs that have mapped from non-standard laboratory test or result codes to LOINC codes, over half reported they have not experienced any issues with LOINC mapping.
Findings
- More than 1 in 5 HIOs reported lacking resources to map or maintain LOINC mapping.
- A smaller portion of HIOs reported insufficient expertise to map to LOINC (10%) and that LOINC tools were too difficult to use (3%).
Figure 5: Issues HIOs report with LOINC mapping from non-standard codes.
![Figure 5 is a horizontal bar chart that shows the percentage of HIO respondents that indicated experiencing a variety of issues with LOINC mapping from non-standard codes. The x-axis displays percentages ranging from 0% to 60%, representing the percentage of HIO respondents, and the y-axis lists each of the following issues (or lack thereof) experienced with LOINC mapping: “Have not experienced any issues”, “Lack resources to map and/or maintain mappings to LOINC”, “Insufficient expertise to map to LOINC within organization”, “Other”, and “LOINC tools too difficult to use.” The horizontal bars displayed indicate that 55% of HIO respondents indicated not having experienced any issues. Meanwhile, 21% reported that they “Lack resources to map and/or maintain mappings to LOINC”, 10% reported that they have “Insufficient expertise to map to LOINC within organization”, 10% indicated that there was some “Other” issue with LOINC mapping from non-standard codes, and 3% reported that “LOINC tools [were] too difficult to use.”](https://healthit.gov/data/wp-content/uploads/sites/2/2024/10/DB75-Figure-5.png?w=1024)
Notes: N=29. 1 missing response was excluded from the denominator. See Appendix Table A1 for survey questions.
Summary
HIOs are commonly used by U.S. hospitals to electronically send and receive summary of care records.(1, 2) The results from this national survey could help inform future standards development and implementation efforts to improve electronic health information exchange. In this brief, we discuss two main types of standards used in health care — content standards and terminology standards. Content standards, such as HL7 v2 and C-CDA, are used to describe the structure and organization of the data, while terminology standards (e.g. LOINC) are used to communicate health concepts using a common set of codes, terms, and classifications.5 In 2023, HIOs widely used document-based exchange to facilitate the sharing of data for its participants. More than 90% of HIOs reported that they routinely or sometimes received and sent (or made available) CDAs. While a majority of HIOs routinely received and sent (or made available) HL7 v2 messages, only a small portion of HIOs reported routinely using HL7 FHIR APIs to receive and send data (or make data available). A majority of HIOs reported receiving HL7 v2 ADT messages, which may be used to enable electronic notifications from hospitals and emergency departments to ambulatory clinicians. Although HIOs have a long history of receiving ADT messages, the high percentage of HIOs receiving HL7 v2 ADT message may also be partly explained by the Centers for Medicare & Medicaid Services’ (CMS) Interoperability and Patient Access final rule’s requirement for the electronic exchange of patient ADT information for psychiatric hospitals, critical access hospitals, and Medicare Conditions of Participation.
In addition to facilitating the movement of data, standards enable encoding of data in a structured format. In the survey, HIOs were asked if they made a list of data elements available to their participating organizations (see Table 1 and Appendix Table A4). As part of our analysis, we mapped these data elements to USCDI versions 1-4 to determine the extent to which HIOs made USCDI-adopted data elements available to participating organizations. Among data elements mapped to the USCDI standard, HIOs, on average, reported making more than two-thirds of these data elements from each USCDI version available to participating organizations. Among all data elements included in the survey, the data elements with the highest rate of HIO availability to participating organizations are immunizations (92%), problems (90%), vital signs (90%), and encounters (90%).
Building on this, we then looked at overall use of the USCDI standard for data received by HIOs from participating organizations and by HIOs when making data available to their members. About 4 in 10 HIOs routinely sent data (or made data available) that adhere to USCDI v1 or v2, and 33% routinely received data from their participants that adhere to USCDI v1 or v2, indicating that HIOs were more likely to send data (or make data available) that adhere to USCDI v1 or v2, but less likely to receive data from participants that adhere to USCDI v1 or v2. Together, these findings show that HIOs are likely to make data elements mapped to USCDI available, but HIOs are less likely to make them available as adhered to USCDI’s semantic standards requirements, and even less likely to receive these data elements from their participants in a standardized manner. Overall, there is a wide range in level of support for standards among HIOs,3 which may explain why many HIOs (around 30% on average) reported not knowing if the information they received or sent (or made available) adhere to USCDI’s semantic standards requirements, indicating a lack of awareness and use of USCDI.
Beyond the general exchange of data, laboratory results that conform to data standards are critical for data precision and quality within EHRs, laboratory information systems, and for public health reporting and surveillance.4 Examining the extent to which HIEs have these data encoded and the barriers associated with this process could inform future efforts to promote laboratory interoperability.5
LOINC codes offer a consistent naming convention for the exchange of laboratory data fields, which minimizes ambiguity in the interpretability of data fields received by a care system. Having a consistent naming convention thus allows data received to be read and integrated properly into electronic health information systems.6 Nearly half (46%) of HIOs reported mapping from non-standard laboratory test or result codes to LOINC codes. This finding suggests that many HIOs may be encountering the use of local codes by laboratories rather than the widespread use of LOINC. Among HIOs that mapped local codes to LOINC codes, a majority did not experience any issues with LOINC mapping but were mapping all, most, or some non-standard laboratory test or result codes to LOINC codes within the past year. However, some HIOs who have mapped from non-standard laboratory test or result codes to LOINC codes reported lacking resources to perform LOINC mapping, having insufficient expertise to map to LOINC codes, or that LOINC tools were too difficult to use. This suggests that available resources might not be adequate to assist most local health care systems in adopting LOINC codes. Our findings demonstrate the need to support laboratories in their use of LOINC and thereby reduce the need for HIOs and other networks to map from local codes to LOINC.
Definitions
HL7 v2 ADT messages: HL7 v2 ADT (Admit, Discharge and Transfer) messages are used to communicate patient demographics, visit information and patient state at a healthcare facility.
Clinical Document Architecture (CDA): A base standard which provides a common architecture, coding, semantic framework, and markup language for the creation of electronic clinical documents.
Continuity of Care Record (CCR): All certified EHRs can generate and receive a Continuity of Care Document (CCD) or Continuity of Care Record (CCR), standard formats for exchanging key clinical information (for example, problem list, medication list, medication allergies, diagnostic test results) among providers of care and patient authorized entities.
Health Information Exchange (HIE): Ability to electronically send or receive patient health information from other providers outside their medical organization using an EHR system or query any patient health information from sources outside of their medical organization.
Health Information Exchange Organizations (HIOs): Local, regional, and state organizations operating in the United States that supported live electronic health information exchange across unaffiliated entities. This does not include local proprietary or enterprise networks.
Interoperability: The ability of a system to exchange electronic health information with and use electronic health information from other systems without special effort on the part of the user.
Logical Observation Identifiers Names and Codes (LOINC Codes): A universal code system for identifying health measurements, observations, and documents. These codes represent the “question” for a test or measurement. LOINC codes can be grouped into Laboratory and Clinical tests, measurements and observations.7,8
United States Core Data for Interoperability (USCDI): A standardized set of health data classes and data elements for nationwide, interoperable health information exchange.9
USCDI Data Class: An aggregation of various data elements by a common theme or use case.9
USCDI Data Element: Piece of data defined in USCDI for access, exchange, or use of electronic health information.9
Data Sources and Methods
Data are from the 2023 National Health Information Organization (HIO) Survey. Since 2020, the Assistant Secretary for Technology Policy/Office of the National Coordinator for Health IT (hereafter ASTP) has partnered with Civitas Networks for Health (Civitas) to examine how HIOs continue to evolve and the role they play in enabling interoperability, as it relates to the broader scope of various policies such as the Health Information Technology for Economic and Clinical Health (HITECH) Act of 2009 and the Trusted Exchange Framework and Common Agreement (TEFCA). ASTP funded the 2023 HIO Survey, led by Julia Adler-Milstein, PhD, Professor and Director of the Division of Clinical Informatics and Digital Transformation at University of California San Francisco (UCSF).
In the survey (see Appendix Table 1 for a list of the relevant questions), HIOs were asked about data elements (see Appendix Table A4) they made available to their members (Q4) and, separately, to what extent they received (Q2) and sent (or made available) (Q5) data using the USCDI standard. In Table 1, we report results from an analysis of these data elements from USCDI versions 1-4, as this analysis preceded the publication of USCDI version 5. The mapping does not reflect all data elements adopted by USCDI versions 1-4 but rather only maps those data elements asked about in the survey to these USCDI versions. Consistent with Executive Order 14168 the sexual orientation, and gender identity data elements have been removed from this data brief. In Figures 2 and 3, we report the results of HIOs’ responses to Q2 and Q5. Responses indicate the extent to which HIOs receive and send (or make available) data using the USCDI standard, specifically, USCDI versions 1 and 2, which, at the time the survey was finalized, were the only published versions of the standard. The two analyses are presented as complementary and present a deeper assessment of HIOs’ use of standards and availability of certain data elements through their networks.
Leadership from 135 HIOs were invited to participate in the survey with the option to complete via the online survey tool Qualtrics© or over the phone if preferred. Non-respondents received follow-up emails and one phone call to encourage response. As with prior years, respondents were offered a $50 gift card for completing the survey or a $10 gift card if they were screened out. 45 HIOs were ineligible for the survey. This brief report results from the 2023 National HIO Survey, which was fielded from January to July 2023. The response rate for national HIOs was 86 percent (N = 77). The responding HIOs operated in 47 states and the District of Columbia.
Data Availability
Data is available upon request. Please contact ASTP_Data@hhs.gov.
References
- Boone KW. Health IT Standards 101: Healthcare IT News; 2012 [Available from: https://www.healthcareitnews.com/blog/health-it-standards-101].
- Parker C, Reeves M, Weiner M, Adler-Milstein J. Health Information Exchange Organizations and Their Support for Research: Current State and Future Outlook. 2017(0046-9580 (Print)).
- The Office of the National Coordinator for Health Information Technology. 2023 Report to Congress. 2024.
- Pylypchuk Y, Everson J. Interoperability and methods of exchange among hospitals in 2021. Washington DC: Office of the National Coordinator for Health Information Technology January 2023.
- Cholan RA, Pappas G, Rehwoldt G, Sills AK, Korte ED, Appleton IK, et al. Encoding laboratory testing data: case studies of the national implementation of HHS requirements and related standards in five laboratories. Journal of the American Medical Informatics Association. 2022;29(8):1372-80.
- Regenstrief Institute. What LOINC is: Regenstrief Institute, Inc.; [Available from: https://loinc.org/about/].
- Regenstrief Institute. Scope of LOINC: Regenstrief Institute, Inc.; [Available from: https://loinc.org/get-started/scope-of-loinc/].
- HIMSS. Interoperability in Healthcare [Available from: https://www.himss.org/resources/interoperability-healthcare].
- The Office of the National Coordinator for Health Information Technology (ONC). United States Core Data for Interoperability. 2020.
Acknowledgments
The authors are with the Office of Technology, within the Office of the Assistant Secretary for Technology Policy and Office of the National Coordinator for Health Information Technology. The data brief was drafted under the direction of Mera Choi, Director of the Technical Strategy and Analysis Division, Vaishali Patel, Deputy Director of the Technical Strategy and Analysis Division, and Wesley Barker, Chief of the Data Analysis Branch with subject matter expertise from Carmela Couderc, Molly Prieto, and Matt Rahn.
Suggested Citation
Chang W, Larson J, Strawley C. Standards Adoption among Health Information Exchange Organizations. Office of the Assistant Secretary for Technology Policy. Data Brief: 75. September 2024.
Appendix
Appendix Table A1: National HIO Survey Questions
| Question Text | Response Options |
|---|---|
| Implementation and Use of Standards | |
| Q1. To what extent does your HIE electronically receive data from your participants using the following approaches listed below? |
For each of the following options:
Respondents were asked to select among these frequency options:
|
| Q2. To what extent is the information that you receive from your participants consistent with different versions of the United States Core Data for Interoperability (USCDI)? |
For each of the following options:
Respondents were asked to select among these frequency options:
|
| Q3. To what extent does your HIE electronically send or make available data to your participants using the following approaches listed below? |
For each of the following options:
Respondents were asked to select among these frequency options:
|
| Q4. Which types of clinical and other health-related information are made available by your HIE (as part of a clinical document or as a structured data element)? (Select all that apply) |
|
| Q5. To what extent does your HIE electronically send or make available to participants |
For each of the following options:
Respondents were asked to select among these frequency options:
|
| Implementation and Use of Standards | |
| Q21. Does your HIE map from non-standard laboratory test or result codes to LOINC codes? |
|
| Q21a. Within the past year, based upon the volume of test results received (qualitative and quantitative), to what extent did your HIE have to map those results from non-standard codes to LOINC codes? |
|
| Q21b. Have you experienced any of the following issues related to mapping to LOINC? (Select all that apply) |
|
Appendix Table A2: Methods by which HIE electronically send or make data available to participants.
| Data Element | Routinely/ From most participants | Sometimes/ From some participants | Rarely/ From few participants | Never | Don’t know |
|---|---|---|---|---|---|
| CDAs | 68% | 25% | 3% | 3% | 1% |
| HL7 v2 messages (any type) | 71% | 12% | 1% | 6% | 10% |
| HL7 FHIR APIs | 15% | 7% | 26% | 38% | 13% |
Notes: N=68. 9 missing responses were excluded from the denominator. See Appendix Table A1 for survey questions. HL7 FHIR messages include respondents who selected HL7 FHIR messages (DSTU2), HL7 FHIR Release 3 (STU) messages, or FHIR v.4.0 messages.
Appendix Table A3: Methods by which HIEs electronically receive data from participants.
| Data Element | Routinely/ From most participants | Sometimes/ From some participants | Rarely/ From few participants | Never | Don’t know |
|---|---|---|---|---|---|
| CDAs | 63% | 27% | 5% | 5% | 0% |
| HL7 v2 messages (any type) | 78% | 10% | 0% | 2% | 11% |
| ADT (for applicable participants) | 90% | 6% | 0% | 3% | 0% |
| HL7 FHIR APIs | 11% | 6% | 24% | 49% | 10% |
Notes: N=63. 14 missing responses were excluded from the denominator. See Appendix Table A1 for survey questions. HL7 FHIR APIs include respondents who selected HL7 FHIR messages (DSTU2), HL7 FHIR Release 3 (STU) messages, or FHIR v.4.0 messages.
Appendix Table A4: Data elements made available by HIOs to participating organizations and the USCDI versions in which each data element is required.
| Data Element | USCDI Versions | % of HIOs |
|---|---|---|
| Data Provenance | 1, 2, 3, 4 | 70% |
| Clinical Information | ||
| Immunizations | 1, 2, 3, 4 | 92% |
| Problems | 1, 2, 3, 4 | 90% |
| Vital Signs | 1, 2, 3, 4 | 90% |
| Prescribed Medications | 1, 2, 3, 4 | 87% |
| Medication Allergies | 1, 2, 3, 4 | 87% |
| Non-Medication Allergies & Intolerances | 4 | 78% |
| Clinical Notes | 1, 2, 3, 4 | 75% |
| Health Concerns | 1, 2, 3, 4 | 66% |
| Family Health History | — | 65% |
| Pregnancy Status | 3, 4 | 62% |
| Functional Status | 3, 4 | 57% |
| Cognitive Status | 3, 4 | 56% |
| Filled Medications | 3, 4 | 52% |
| Imaging/Pathology | ||
| Radiology Report (narrative) | 1, 2, 3, 4 | 86% |
| Pathology Report (narrative) | 1 only (removed from subsequent USCDI versions) | 84% |
| Diagnostic Imaging Order | — | 51% |
| Laboratory-Related Information | ||
| Laboratory Value(s)/Results | 1, 2, 3, 4 | 84% |
| Laboratory Test | 1, 2, 3, 4 | 81% |
| Laboratory report (Narrative) | 1 only (removed from subsequent USCDI versions) | 75% |
| Team-Based Care | ||
| Care Team Members | 1, 2, 3, 4 | 61% |
| Assessment and Plan of Treatment | 1, 2, 3, 4 | 60% |
| Care Plan Fields | 1, 2, 3, 4 | 51% |
| Encounter-Related Information | ||
| Encounters (encounter type, diagnosis, time) | 2, 3, 4 | 90% |
| Procedures | 1, 2, 3, 4 | 88% |
| Admission and Discharge Dates and Locations | 1, 2, 3, 4 | 87% |
| Reason for Hospitalization | 1, 2, 3, 4 | 82% |
| Discharge Disposition | 2, 3, 4 | 82% |
| Discharge Instructions | 1, 2, 3, 4 | 74% |
| Referrals | 1, 2, 3, 4 | 65% |
| Other Data Elements | ||
| Home Address | 1, 2, 3, 4 | 84% |
| Race/Ethnicity | 1, 2, 3, 4 | 82% |
| Preferred Language | 1, 2, 3, 4 | 68% |
| Health-Related Social Needs | 2, 3, 4 | 52% |
| Substance Use Disorder | 4 | 39% |
| Sexual Orientation | 3, 4 | 35% |
| Other | N/A | 12% |
Notes: This table reflects only USCDI elements included in survey question Q4. See Appendix Table A1 for question text. N=77; the numerator includes only those HIOs that make all data elements from each respective USCDI version available, among those elements included in Q4 of the National Survey of Health Information Exchange Organizations. For our analysis, we mapped these data elements to USCDI versions 1-4. USCDI v5 was published in July 2024 (after analysis was completed for this brief) and was therefore not included in this brief’s analysis and mapping. See Methods section for more details.
- The Office of the National Coordinator for Health Information Technology. 2023 Report to Congress. 2024.
- Pylypchuk Y, Everson J. Interoperability and methods of exchange among hospitals in 2021. Washington DC: Office of the National Coordinator for Health Information Technology January 2023.
- Everson J, Chang W, Patel V, Adler-Milstein J. The state of health information organizations and plans to participate in the federal exchange framework. Health Affairs Scholar. 2024;2(8).
- Cholan RA, Pappas G, Rehwoldt G, Sills AK, Korte ED, Appleton IK, et al. Encoding laboratory testing data: case studies of the national implementation of HHS requirements and related standards in five laboratories. Journal of the American Medical Informatics Association. 2022;29(8):1372-80.
- Strawley C. L, J., Patel, V. Laboratory Interoperability Through Health Information Exchange Organizations. Washington, D.C.; 2024.
- Regenstrief Institute. What LOINC is: Regenstrief Institute, Inc.; [Available from: https://loinc.org/about/.
